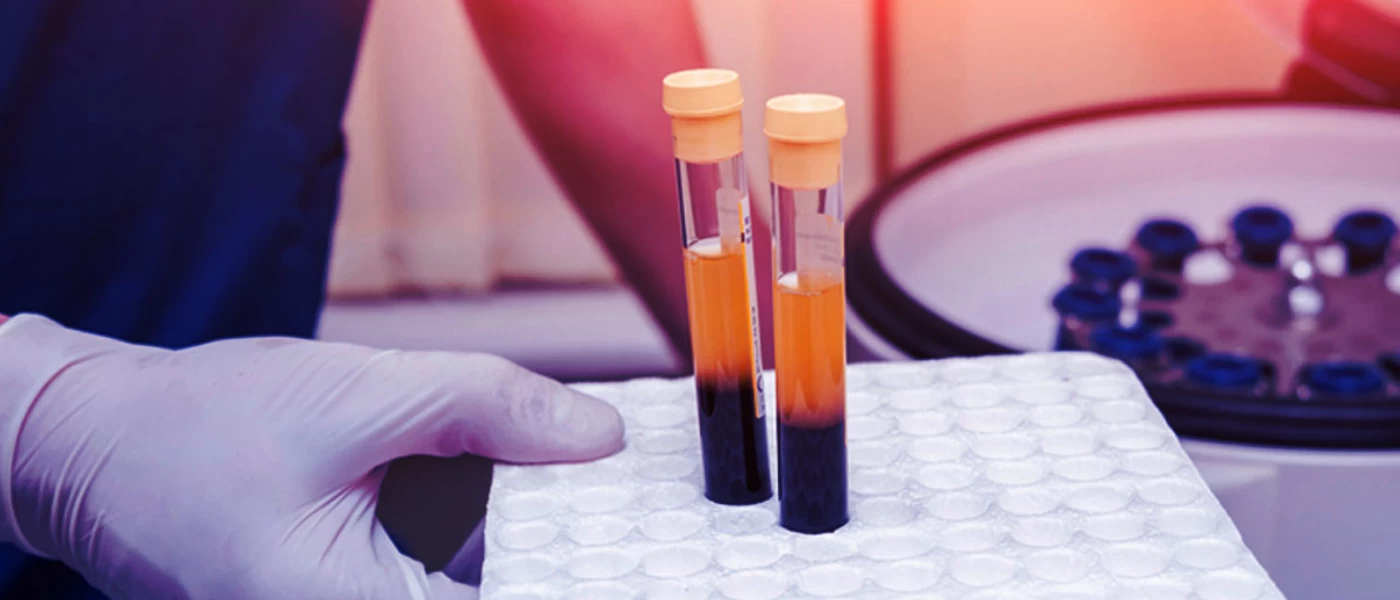
Indian Council of Medical Research (hereinafter referred as the ‘ICMR’) has recently come up with letter of intent inviting hospitals and institutions to participate in randomized controlled study of Therapeutic Plasma Exchange in critically infected COVID-19 patients. This is also being referred to as the ‘Convalescent Plasma Therapy’. The interested institutions can provide treatment to patients on the lines of Clinical Trials protocols that too if the procedure of treatment is cleared by Institutional Ethics Committee.
The ‘Convalescent Plasma Therapy’ is being developed globally to treat critically ill patients of COVID-19. The R & D in this area of medicine has already started in many countries; however, problems are being faced by researchers globally which is either due to restriction imposed on use of ‘blood’ for research or lack of specific regulation/guidelines on collection of whole blood from recovered COVID-19 patients. In India, ICMR has showed interest in this therapy treatment and therefore, asked Hospitals and Institutions to come forward to participate in carrying out the Clinical Trial study which will include patients critically infected by COVID-19. However, the treatment/study will require collection of ‘blood plasma ’from recovered patients of deadly COVID-19 infection.Here, the need of well-documented procedure/guideline for collection of blood from recovered patient will arise.
Here, the point of concern is the lack of comprehensive blood regulation is troubling researchers not only in India but globally and the authorities must have felt this by now.
It is important to highlight that illegal sale of ‘Blood’ has been a major concern in India for decades and in absence of separate regulation/act governing ‘Blood’, the illegal sale may continue to pose threat to the new development of science. Therefore, it is high time to ponder on the need of a separate regulation/act on Blood which will help to tackle the illegal sale menace.
Blood and all components derived from it are ‘Drugs’ as per the definition of drug under section 3(b) of Drugs and Cosmetics Act, 1940 (hereinafter referred to as ‘the Drugs Act’). The licensing, manufacturing, sale, distribution and storage of it are regulated by the Drugs Act and Rules and Regulations made thereunder. The Drugs and Cosmetics Rules, 1945 (hereinafter as ‘the Drugs Rules’) has laid down guidelines for collection of blood from donor who fulfill certain conditions.Further, it also prohibits collection of blood from ‘professional donors’- a donor who donates in lieu of consideration for money or in-kind barring certain defined exclusions. The illegal sale of blood was brought to the notice of apex court in the case of Common Causev. Union of India and Others [Writ Petition (Civil) 91 of 1992] and the hon’ble Supreme Court was apprised of the illegal red market operating in India through various unlicensed blood banks selling blood at exorbitant prices. It was also pointed out that the blood was not being properly collected, tested and stored&as a result, it posed a threat to patients receiving it. The court directed the Indian government to come up with nationwide programme to curb this menace. In response, the Indian government entrusted M/s A.F. Ferguson and Co. with the task to study blood banking system in India.
The effect of the conclusion derived from the study helped to streamline the collection and transfusion of blood through formation of some policies and guidelines. However, the situation did not improve very well as some of the survey reports suggest that the professional donor and illegal red markets are still operative. The reason of this may be the lack of awareness about positive benefits of blood donation and lack of insufficient voluntary donors. The root cause of these problems is lack of well drafted separate regulation/act governing blood and absence of a sole regulatory body to exclusively deal with every aspect of ‘blood’.
The most important concern which arises due to absence of comprehensive blood regulations is the growing importance as well as requirement of blood for research and development purposes by the Pharmaceutical Industries. R&D is an important aspect of drug development especially in emerging technologies of treatment using blood components like plasma therapy, hyper immunoglobulins etc. The research on this emerging technology requires the initiating substance i.e. blood; specially from those who have recovered from a deadly disease like COVID-19. In order to deal with present public health emergency, US FDA has issued guidance to provide recommendations to health care providers and investigators on the administration and study of investigational convalescent plasma collected from individuals who have recovered from COVID-19.The guidance provides recommendations on following: -
- pathways for use of investigational COVID-19 convalescent plasma
- patient eligibility
- collection of COVID-19 convalescent plasma, including donor eligibility and donor qualifications;
- labelling; and
- record keeping
- Taking clue from the guidance note issued by US FDA, the Indian regulators should soon take concrete steps in formalizing a well drafted blood regulation to tackle all the existing problems and confusions as on date.
[The authors are Partner and Associate, respectively, in Food Law practice of Lakshmikumaran& Sridharan, New Delhi]