Introduction
The Intellectual Property Appellate Board (‘IPAB’) in a recent order dated 29-09-2020[1] allowed the appeal filed by Pharmacyclics, LLC (‘Appellant’) challenging the revocation of Indian Patent No. 262968[2] (‘IN’968’) by the Joint Controller of Patents and Designs, New Delhi[3] (‘Respondent No. 2’) following post-grant opposition proceedings initiated by Laurus Labs Pvt. Ltd. (‘Respondent No.3); and has set aside the revocation order (‘impugned order’) as being ‘devoid of merit’. Consequently, the IPAB directed the Controller General of Patents, Designs, Trademarks and Geographical Indications (‘Respondent No. 1’) to take immediate steps to make necessary changes in the records and the e-register pertaining to IN’968. The Respondent No. 2 revoked IN’968 only on the ground of lack of inventive step and the same has been overturned by the IPAB in appeal.
In the instant order, the IPAB has delineated some important legal principles, especially in the context of inventive step assessment under Section 2(1)(ja) of the Patents Act, 1970 (‘Act’) which will help to make the adjudication with respect to inventive step more objective.
Prior to the instant order on merits, the IPAB also dealt with a miscellaneous petition filed by the Appellant for interim stay of the impugned order during the pendency of the appeal. The IPAB vide Order dated 12-06-2020 had issued an ad-interim stay on the impugned order. After an unsuccessful challenge of the said ad-interim order by the Respondent No. 3 before the Delhi High Court in a writ proceeding[4], the IPAB vide Order dated 07-08-2020 decided the application for stay in favour of the Appellant, essentially owing to procedural lapses during the post-grant opposition proceedings and for not adhering to the guidelines issued by the Delhi High Court in this regard. It is pertinent to note that by Order dated 20-11-2019, the Delhi High Court in a writ proceeding[5] initiated by the Appellant challenging the Respondent No. 2’s decision to allow additional documents and evidence filed by the Respondent No. 3 to be taken on record, laid down the general principles that ought to be followed while dealing with a post-grant opposition. It was owing to the non-compliance of the said principles that the stay of the impugned order was granted by the IPAB.
Facts leading up to the impugned order
The Appellant’s IN‘968 was granted on 25-09-2014 with claims 1-2. The claims cover, amongst other compounds, a compound having an International Non-Proprietary Name (INN), IBRUTINIB[6] (IMBRUVICA ®), relating to irreversible Bruton Tyrosine Kinase (‘Btk’) Inhibitors that is useful for the treatment of disease associated with B-cell malignancies.
The Respondent No. 3 instituted post-grant opposition proceedings against IN’968 on 24-09-2015 on the grounds of lack of novelty, inventive step, sufficiency, for falling under the scope of Section 3(d) of the Act and also filed the evidence of Dr. CH. V. Ramana Rao, Head IP Management of the Respondent No. 3 in support thereof. Thereafter, the Appellant filed the reply to the post-grant opposition on 23-12-2015 along with the evidence of their independent expert, Dr. Alexander James Bridges. The Respondent No. 2 constituted the Opposition Board under Section 25(3)(b) of the Act and Rule 56 of the Rules and forwarded the documents to the said Board on 14-09-2017. The Opposition Board evaluated the documents and evidence filed by both the parties under Rules 57 to 60 of the Rules and submitted their recommendation to Respondent No. 2 under Rule 56(4) of the Rules on 23-03-2017. The Board recommended that none of the grounds of the opposition was established and therefore recommended that IN’968 be maintained.
Pursuant thereto, the hearing was fixed by the Respondent No. 2 on 16-11-2017. However, following an adjournment request by the Respondent No. 3, the next date of hearing was scheduled to be held on 25-09-2019. Ahead of the said hearing, the Respondent No. 3 filed further documents on 12-09-2019 and filed a petition under Rule 137 of the Patents Rules, 2003 (‘Rules’) on 19-09-2019 seeking permission to file further evidence under Rule 62(4) of the Rules in the form of an Affidavit by Dr. Boyapati Manoranjan Choudhary. The Appellant thereafter sought adjournment of the hearing and subsequently filed petitions under Rule 137 of the Rules dated 11 and 14-10-2019 praying that the documents and evidence filed by the Respondent No. 3 ought not to be considered and that this limited issue ought to be decided ahead of the main opposition itself. The Respondent No. 3 duly filed responses to the said petitions on 16-10-2019. Vide order dated 06-11-2019; the Respondent No. 2 allowed the petition filed by the Respondent No. 3 and consequently took on record the additional documents and evidence by way of affidavit filed by the Respondent No. 3. Thereafter, the Appellant on 15 & 18-11-2019 filed a petition under Rule 128 of the Rules read with Section 25(2) of the Act and Rules 60 / 62(4) of the Rules seeking permission to file an Affidavit of Dr. Alexander Bridges and filed the said affidavit.
The Respondent No. 2 conducted the hearing on merits in the opposition on 22-11-2019 and thereafter vide impugned order dated 04-03-2020 held that the grounds of lack of novelty, lack of sufficient description and non-patentability under Section 3(d) of the Act are not maintainable. However, the Respondent No. 2 also held that IN’968 is liable to be revoked for lack of inventive step under Section 2(1)(ja) of the Act in the light of the cited prior art documents[7]. The Controller also adjudicated some preliminary issues raised by the Appellant, as follows:
- That the written submissions filed by the Respondent No. 3 are beyond the pleadings and evidence filed by the Respondent No. 3 and is an attempt to introduce a new post grant opposition- The Respondent No. 2 held that no new case has been made out and also noted that pursuant to orders of the Delhi High Court, the Appellant was given an opportunity to rebut the additional documents and evidence.
- Evidence of Dr. Boyapati Manoranjan Choudhary ought not to be entertained as it goes beyond the pleadings filed by the Respondent No.3- The Respondent No. 2 held that sufficient opportunity was given to the Appellant to respond to any grounds, considered new by the Appellant. The Respondent No. 2 also noted that the Appellant filed its own evidence is response to the evidence by the Respondent No. 3 and that the Delhi High Court directed that all the evidence produced by both parties shall be considered.
- Evidence of Dr. Ramana Rao on behalf of the Respondent No. 3 ought not to be considered as he works for the Respondent No. 3 and is not an independent expert- The Respondent No. 2 held that only the technical aspects of the said affidavit were being considered, against which no objection was raised by the Appellant and that in any case, the Respondent No. 3 has also filed the evidence by way of affidavit of Dr. Choudhary.
In revoking IN’968 for lack of inventive step, the Respondent No.2 held as follows:
Even though all the cited prior art documents pertain to Lck inhibitors as opposed to the claimed compound in IN’968 which pertains to Btk inhibitors, the cited prior art documents are relevant because they pertain to proteins under the general tyrosine kinase family. It was held that there exists homology between the two classes of inhibitors owing to similar structure. The Respondent No. 2 thus held Lck and Btk analogous primarily for two reasons i.e. allegedly admitted facts in the compete specification of IN’968 and the views expressed by the Respondent No. 3 on the alleged admitted facts of complete specification of IN’968, US’851 and Chen et al[8].
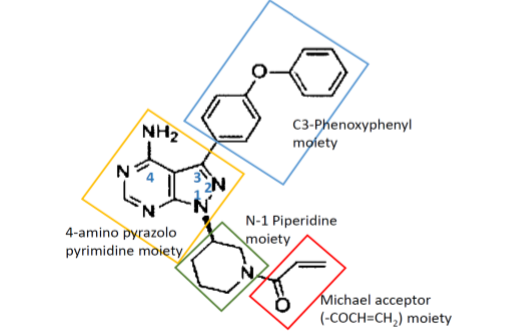
The Respondent No. 2 relied on the disclosure in Andrew et al.[9] to conclude that a person skilled in the art would be motivated to select a structure with a pyrazolopyrimidine core (Compounds 1 and 2 disclosed therein) and that the person skilled in the art would also know based on the said disclosure that pyrazolopyrimidine core is more active than pyrolopyrirridine core. This is relevant because the claimed compound Ibrutinib of IN’968 has a pyrazolo pyrimidine scaffold with the following substituents-
- N-1 position with substituted piperidine ring (‘substituent a’),
- C-3 position with phenoxy phenyl group (‘substituent b’) and
- C-4 position with amino group
- Michael acceptor (-COCH=CH2 group) attached at N-1 position on piperidine ring at the 3rd position[10] (‘Michael acceptor’).
With respect to the ‘substituent b’, the Respondent No. 2 held that as per the disclosure in Andrew et al., this group at this position increases potency and that the Appellant failed to give any reasons as to why this logical substitution would not have been made by a person skilled in the art.
With respect to the ‘substituent a’, the Respondent No. 2 acknowledged that the compounds in Andrew et al. have a cyclohexyl group at 1st position and to that is appended N-methyl piperazine. The Respondent No. 2 noted that as per Andrew et al., an appended solubilizing heterocycle is in the ribose pocket, such as the N-methyl piperazine at 1st The Respondent No. 2 also duly noted that the compounds disclosed in Andrew et al. have a cyclohexyl group at 1st position and to that N-methyl piperazine is appended, whereas the claimed compound Ibrutinib of IN’968 has piperidinyl group. However, the Respondent No. 2 reasoned that a person skilled in the art when preparing a new compound would look for similar Nitrogen based heterocyclic groups which are structurally similar to cyclohexyl group and that could similarly occupy the ribose pocket as N-methyl piperazine. The Respondent No.2 further reasoned that one such similar group is the piperidinyl group used in the prior art document WO’868. The Respondent No. 2 further held that in chemistry, there are only few aza-heterocyclic groups and piperidinyl is not uncommon. The Respondent No. 2 thus held that since piperidinyl group seems to be used in the prior art compounds and such compounds of prior art do behave as tyrosine kinase inhibitors, hence one can expect a replacement at the 1st position with piperidinyl group and expect anti-tyrosine kinase activity and this will be obvious to a person skilled in the art.
With respect to the ‘Michael acceptor’, the Respondent No. 2 relied on the Copeland[11]article which proposes the strategy of making irreversible inhibitors for many proteases as well as kinase targets. The Respondent No. 2 reasoned that based on the disclosure in Copeland, adding Michael acceptor to the main compound was possible and success could be achieved (examples EKB-569 and CI-1033) and therefore there is sufficient basis for a person skilled in the art to adopt this strategy. The Respondent No. 2 further reasoned that a person skilled in the art would attach a Michael acceptor from Copeland to the hypothetical compound from Andrew et al. and that this modification is not a major one as there are steps already stated in the art and it only has to be followed. The Respondent No. 2 specifically pointed out that if one were to peruse the compound disclosed in Andrew et al. there is only one position available for further substitution, the nitrogen at the free end of the piperidinyl group and that the Michael acceptor cannot be added to the 3rd position as it is occupied by phenoxy-phenyl group. Thereafter, the Respondent No. 2 also held that it would be natural for a person ordinarily skilled in the art to try and use vinyl ketone as a moiety to give the irreversible inhibitor effect.
Based on the above reasoning and by relying upon the decision in Bishwanath Prasad Radhey Shyam v. Hindustan Metal Industries [AIR 1982 Supreme Court 1444], the Respondent No. 2 held that the cited documents clearly disclose or teach all the features of the claimed invention and the invention is merely a combination of known features, which does not give rise to an inventive technical advance. It was also held that for a person ordinarily skilled in the art, these are obvious modifications to make and if made, the said person could expect the compound coming out to have tyrosine kinase activity, especially against Lck and therefore, IN’968 lacks inventive step.
Importantly, the Respondent No. 2 held that the Opposition Board had not considered all the matters in this much detail and therefore the Respondent No. 2 did not agree with the recommendation of the Opposition Board. The Respondent No. 2 also noted that after the recommendations of the Board were made, there were further affidavits filed by both parties, but the same was not sent to the Board as this would delay the proceedings by at least 6 months. The Respondent No. 2 also held that this is not necessary as per the procedure under the Act and the Rules and also that none of the parties have requested for the same. Additionally, the Respondent No. 2 noted that although the Respondent No. 2 has seen all the judgments referred to by both parties, the Respondent No. 2 has not made specific mention of each of the judgments because the matter is more factual.
Arguments by the appellant and respondent no. 3
The Appellant mainly asserted the following against the impugned order:
The Respondent No. 2 completely disregarded the directions of the Delhi High Court in respect of post-grant opposition guidelines, specifically with respect to the direction that the Respondent no. 2 is to ensure that the members of the Opposition Board are present at the hearing so that new document/evidence is discussed in the presence of the Opposition Board. Accordingly, the Respondent No. 2 erred in not sending the new documents and fresh evidence to the Opposition Board for fresh Opposition Board recommendations.
The Respondent No. 2 disregarded the recommendations of the Opposition Board without any cogent reasoning.
The Respondent no. 2 ignored the well settled principle for obviousness determination which is a mixed question of law and fact.
The Respondent No. 2 erred in not deciding/adjudicating upon the preliminary objections taken by the Appellant.
The Respondent No. 2 erred in appreciating the evidence of Dr. Alex Bridges as well as the details provided in the specification of IN’968 that clearly recognize that Btk and Lck are different kinases and there is no similarity in the two kinases whatsoever. The Appellant asserted that the complete specification of IN’968 shows different types of receptor/non-receptor tyrosine kinases and that Btk contains Cysteine at 481 position, while Lck contains Serine amino acid. Thus, Lck (and Lyn and Syk) does not have a cysteine residue in the kinase domain, let alone a cysteine residue corresponding to the 481 position of Btk. Thus, it was asserted that Lck is not Btk homolog or Btk cysteine homolog.
The findings of Respondent No. 2 in the inventive step analysis are incorrect on facts and comprises major scientific errors which were neither argued at the hearing, present in the hearing submissions nor in the evidence of their experts. The Appellant asserted that the act of the Respondent No. 2 to equate the “1-methyl-piperazin-4-yl-cyclohexyl” moiety to be interchangeable with a “piperidine” ring is without any scientific reasoning and is against the basic principles of medicinal chemistry. It was asserted that these two groups are structurally and functionally different and one cannot use them as interchangeable in the absence of any empirical studies. It was argued that Medicinal chemistry is an unpredictable art and a small change in the structure of a compound can have drastic effect on its activity. The Appellant asserted that replacing one group with another (piperidine for cyclohexyl appended with N-methyl piperazine) could lead to change in the compound's electronic and steric properties and binding to the active site (Lck) and that it would be entirely unpredictable whether making such a modification would produce anti-tyrosine kinase activity or not.
The Appellant also asserted that a person ordinarily skilled in the art would be motivated to use and retain the piperazine ring appended onto the cyclohexyl group as taught in Andrew et al. and would not be motivated to remove the N-piperazinyl group as it has provided the very advantage the Andrew et al. ascribes to it. The Appellant asserted that this would teach away from removing the piperazinyl group. Accordingly, without a motivation to change the N-piperazinyl-cyclohexyl group a person ordinarily skilled in the art would not change that group.
The Respondent No. 2 erred in arbitrarily selecting prior art documents to show the various substituents as that in the claimed compound Ibrutinib of IN’968 and thus indulged in hindsight analysis. The Appellant asserted that there is no motivation to attach a Michael acceptor from the prior art Copeland to a hypothetical compound, which the Respondent No. 2 has failed to identify, from Andrew et. al. and other prior art references. The Appellant argued that the arbitrary selection of the hypothetical compound from Andrew et al., and attaching a Michael acceptor from Robert Copeland to the said compound, is a radical modification of the hypothetical compound and not just a "minimum change" as pointed out by the Respondent No. 2 in the impugned order. The Appellant asserted that this would lead to a radical change in the hypothetical compound’s mechanism of action, binding in the active site, electronic and steric properties as well as introducing potential off-target binding, glutathione binding and other possible negative effects.
The Appellant argued that the Respondent No. 2 arbitrarily arrived at the conclusion that the Michael acceptor cannot be added to the 3rd position as it is occupied by phenoxy-phenyl group and provided no basis for the selection of “vinyl ketone”, to use as a Michael acceptor, since the complete specification of IN’968 provided a laundry list of Michael acceptors. Additionally, it was also pointed out that there is no teaching to incorporate the Michael acceptor on the nitrogen atom of the piperidinyl ring and not on the 4-amino group. The Appellant also emphasized that there is teaching away from using Michael acceptors as it causes immunogenicity toxicity and instability. The Appellant asserted that the Respondent No. 2’s reliance on the molecule CI-1033 was misplaced as it is distinctly dissimilar to that of Ibrutinib. The former being EGFR inhibitor (receptor tyrosine kinase) and Ibrutinib being a Btk inhibitor (non-receptor tyrosine kinase).
The Respondent No. 3 mainly asserted the following in support of the impugned order:
- It is settled law that the recommendations of the Board are not binding, are merely recommendatory in nature. In view of Section 25(3) of the Act as well as Rule 56 of the Act, the recommendation of the Opposition Board is a mere recommendation and is not binding on the Respondent No.2, who is free to adjudicate the matter on its own merits, independent of the recommendations of the Board.
- The Respondent No. 3 argued that the scheme of the Act does not contemplate that all the members of the Opposition Board should be present necessarily at the hearing. It was argued that the Board after giving its opinion becomes functus officio and has no role to play and therefore even if the members were required and not present, it is a mere irregularity.
- The Respondent No. 2 rightly held that Lck is homologous to Btk because the main binding domain is the ATP binding domain, which is common to Lck and Btk enzymes and both relate to tyrosine kinase enzymes that are involved in mediating cancer cells. Therefore, all prior art documents relating to Lck are analogous prior art documents.
- The Respondent No. 2 has not committed any scientific inaccuracies and that the Appellant’s have resorted to selective reading and failed to consider the impugned order in its entirety.
- The Respondent No. 2 has not indulged in any hindsight analysis and has logically followed the prior art disclosure and read the prior art collectively as a person skilled in the art would do. It was also asserted that all chemical substances are built on using a common core and that it is this core that provides the intended effect. Therefore, compounds having similar core have similar properties. The Respondent No. 3 asserted that Copeland discloses that compounds produced by incorporation of Michael acceptors to any known drug molecule, in order to covalently inactivate cysteine residues in their target enzymes have been clinically tested[12] and found to be effective irreversible inhibitors and thus the addition of Michael acceptor to form Irreversible Inhibitor is obvious.
IPAB’s reasoning
Post-grant opposition procedure
The IPAB perused the relevant provisions pertaining to the post-grant opposition procedure under the Act and Rules and held that whatever evidence needs to be filed by either party, whether under Rules 57-59 of the Rules or Rule 60 of the Rules, it must be filed prior to the date of hearing as fixed under Rule 62 of the Rules. It is implied that after the date of hearing is fixed no evidence by either party is admissible as per the provisions of the Act and Rules made thereunder. The IPAB also held that the above applies irrespective of any adjournments of the hearing, that is, the relevant date of hearing for assessing admissibility of evidence is the date of first hearing. The IPAB also noted that the only limited exception to the above is under Rule 62(4) of the Rules which states that if either party intends to rely on any publication in the public domain at the hearing, which is not already mentioned in the notice, statement or evidence, then such a party shall give to the other party and to the Controller not less than five days' notice of his intention, together with details of such publication.
The IPAB further noted that the Act and the Rules, in the context of post-grant opposition, contemplate a two-tier decision-making process wherein the opposition board under a chairman examines all the statements and evidences and submits its recommendation to the Controller and thereafter, the Controller considers the said recommendation and finally adjudicates the matter. The IPAB thus held that although the Opposition Board’s recommendation is not binding on the Controller, it is mandatory for the Controller to take the recommendation of the Opposition Board into consideration. The IPAB held that the reasons for the Controller’s agreement or disagreement with the recommendation of the opposition Board, are required to be annotated.
In the instant appeal, the IPAB noted that a number of documents were placed on record by the Respondent No. 3 under Rule 62(4) of the Rules and the Appellant also filed their rebuttal to the same thereafter. The IPAB held that this was not in the spirit of the Rules. The IPAB reasoned that if the legislature thought that the ‘publication’’ under Rules 62(4) of the Rules has evidentiary value, then there was no logical reason to exclude the Opposition Board from analysing such publications and instead limiting the Board to only the documents submitted under Rules 57-60 of the Rules. The IPAB held that the intention of the legislature was clear that the publications under Rule 62(4) of the Rules were never to be equated on par with documentary evidence which were required to be examined for veracity and merit by the Opposition Board and hence did not require examination by the Opposition Board. The IPAB also reiterated the guidelines issued in this regard by the Delhi High Court.
Applying the above-mentioned principles in the instant case, the IPAB held that the additional documents and evidence, albeit not in consonance with the provisions of the Act and Rules, were rightly considered by the Respondent No. 2 in the instant case as the same were allowed by the Delhi High Court in the writ proceedings. However, the IPAB noted that the lack of presence of all members of the Opposition Board during the hearing before the Respondent No. 2 and not sending the additional documents and evidence to the Opposition Board were violative of the provisions of the Act and Rules and the principles as laid down by the Delhi High Court. The IPAB also held that in the instant case when the Respondent No. 2 disagreed with the recommendations of the Opposition Board, he ought to have annotated the reasons for disagreement properly.
Determination of inventive step
The IPAB considered the relevant statutory provisions, the relevant case law and explanation provided under the Patent Office Manual. The IPAB proceeded to adjudicate the issues pertaining to inventive step as follows:
- The IPAB held that nowhere in the complete specification of IN’968 are both Lck and Btk shown as analogues. It was held that Lck does not share homology with Btk as it does not have a cysteine residue at 481 position rather Lck contains Serine at the corresponding 481 position and not the Cysteine amino acid residue. The IPAB reasoned that for inventive step determination the prior arts should be analogous. It was held that in order for a reference to be proper for use in an obviousness rejection, the reference must be analogous art to the claimed invention. The IPAB held that the prior art documents cited by the Respondent No. 3 are not analogous and any determination of inventive step based upon the non-analogous prior arts will not yield proper result.
- The IPAB concurred with the Appellant’s arguments that there was no reason for a person skilled in the art to:
- select compound 2 from Andrew et al. for a Lck for irreversible Btk inhibitor,
- modify the compound 2 of Andrew et al. by removing the most critical part of the molecule despite the teaching of the said document being to modify the 3’ position of the pyrrolopyrimidine/ pyrazolopyrimidine ring,
- select piperidine ring let alone 3’ piperidine ring as present in Ibrutinib,
- attach a Michael acceptor of EGFR (a Receptor tyrosine kinase inhibitor) to a hypothetical molecule which is a Lck inhibitor to arrive at an irreversible Btk inhibitor (a non-receptor tyrosine kinase).
The IPAB held that the above substitutions are somehow trying to trace back to the invention by keeping the invention in forefront and it amounts to ‘hindsight analysis’. The IPAB held that the combination of the teachings in the cited prior arts documents failed to reach at a compound which selectively inhibits Btk. The IPAB held that contrary to established jurisprudence, in the instant case, every substitution alleged by the Respondent No. 2 to be taken by the person skilled in the art is done to reach to the invention having full knowledge of the invention and thus is legally untenable.
The IPAB held that the Respondent No. 2 failed to appreciate that the determination of “inventive step’ is mixed question of law and facts depending largely on the circumstances of the case and therefore the Respondent No. 2 ought not to have ignored the legal aspects when determining inventive step.
The IPAB held that while the Respondent No. 2 relied on the decision of the Hon’ble Supreme Court in Bishwanath Prasad Radhey Shyam Appellant Hindustan Metal Industries [AIR 1982 Supreme Court 1444] and concluded that the claimed invention consists merely a combination of known features, which does not give rise to an inventive technical advance; the Respondent No. 2 failed to adjudicate whether this combination is more than a mere workshop improvement or whether the new combination satisfies the test of inventiveness on its own.
The IPAB noted that the Respondent No. 2 held that the granted claims are obvious to an ordinary person skilled in the art and therefore lacks inventive step over cited prior art documents. The IPAB, in this regard held that the concept of ‘ordinary’ person skilled in the art is not available under the Act. The IPAB clarified that the determination of ‘inventive step’ as envisaged under Section 2(1)(ja) of the Act clearly stipulates ‘person skilled in the art’. The adjective ‘ordinary’ does not find mention with ‘person skilled in the art’ in the Act. The IPAB also noted that the concept of ‘person skilled in the art’ is often confused with the concept of ‘a person in India possessing average skill in, and average knowledge’ as provided under Section 64(h) of the Act. The IPAB explained that the latter is for determining the ‘sufficiency of disclosure’ by proving ‘workability’ of invention and it is different than that of ascertaining the patentability requirements such as determination of ‘inventive step’ of an invention which requires ‘person skilled in the art’.
Conclusion
The instant IPAB order provides some important pointers for inventive step assessment, especially for inventions in the pharmaceutical sector; such as the necessity to assess whether cited prior art documents are analogous in nature and the necessity to identify the motivation in the prior art for a person skilled in the art to undertake substitutions in prior art compound(s) without having knowledge of the claimed invention and taking into account any ‘teaching away’ in the prior art documents.
Although the tests for inventive step assessment were firmly established by the Division Bench of the Delhi High Court back in 2015 in Roche v. Cipla [2016 (65) PTC 1 (Del)], there was a dearth of practical pointers for the application of those tests to inventions in various fields of technology, thus making the assessment very subjective. Clearly, the task of providing such pointers can only be fulfilled by the IPAB which is uniquely equipped to adjudicate matters from both a legal and technical perspective. With the recent and robust functioning of the IPAB, more such orders can be expected and in relation to various fields of technology which may eliminate needless inconsistencies in Controller’s orders and ensure that the patentability assessment is consistent overall.
[The authors are Principal Partner and Joint Partner, respectively, in Intellectual Property Rights team in Lakshmikumaran & Sridharan Attorneys]
- [1] OA/46/2020/PT/DEL dated 29-09-2020.
- [2] Patent Application no. 1642/DELNP/2009, titled "INHIBITORS OF BRUTON'S TYROSINE KINASE”.
- [3] Order revoking Patent No. 262968 dated 04-03-2020.
- [4] WP(C) 3582/2020 dated 17-06-2020.
- [5] W.P.(C) 12105/2019 dated 20-11-2019.
- [6] Ibrutinib is specifically disclosed as Compound 13 (R)-1-(3-(4-amino-3-(4phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl)piperidin-1-yl)prop-2-en-1one.
- [7] WO2002/080926; US2004/0006083, WO2004/100868 (WO’868), Andrew F. Burchat et. al., Bioorganic & Med. Chemistry Letters, 2002, 12, 1687-1690 (Andrew et al.), Robert A. Copeland, Evaluation of Enzyme Inhibitors in Drug Discovery, 2005 (Copeland), US 2005/0196851 (US’851) and Chen Mao, et. al.; The J. Biol. Chemistry, 2001, Vol. 276(44)(2) page 41435-43 (Chen et al.).
- [8] Supra Note 7
- [9] Supra Note 7
- [10]
- [11] Supra Note 7
- [12] The Respondent No. 3 specifically relied upon EKB-569 and Cl-1033 as disclosed in Copeland.












